Wearable ketamine delivery device complements PharmaTher’s ketamine product portfolio, including injectable, intravenous, and microneedle patch
PharmaTher aims to become a leader in specialty ketamine products for mental health, neurological and pain disorders
TORONTO, May 25, 2022 -- PharmaTher Holdings Ltd. (the “Company” or “PharmaTher”) (OTCQB: PHRRF) (CSE: PHRM), a company focused on the development and commercialization of specialty ketamine products, and CC Biotechnology Corporation (“CCBIO”), a leader in the design and manufacturing of wearable, pen and auto injectors, today announced they have entered into a development agreement to combine PharmaTher’s ketamine formulation with CCBIO’s Felice Dose wearable delivery device to create a proprietary wearable ketamine delivery solution for mental health, neurological and pain disorders. The Company expects to conduct clinical studies with its wearable ketamine delivery device in Q1-2023.
Currently, ketamine is delivered predominately by intravenous (“IV”) and intramuscular (“IM”) means in a controlled healthcare setting (i.e. hospital or clinic) for analgesia, sedation and anesthetic induction and is also emerging as a viable treatment option for various mental health, neurological and pain disorders. The Company believes that in the natural evolution of chronic disease management, a logical progression from IV and IM to wearable injection systems and microneedle patches could increase the convenience, compliance, and dose flexibility for both caregivers and patients.
PharmaTher’s proposed wearable ketamine delivery device aims to convert IV and IM delivery of ketamine to a subcutaneous format, which may improve safety and efficacy, reduce side effects, enhance patient comfort and adherence, and reduce the time burden of treatment on patients and healthcare providers whether at hospital, clinic or home, and therefore may expand the adoption of ketamine for unmet medical needs.
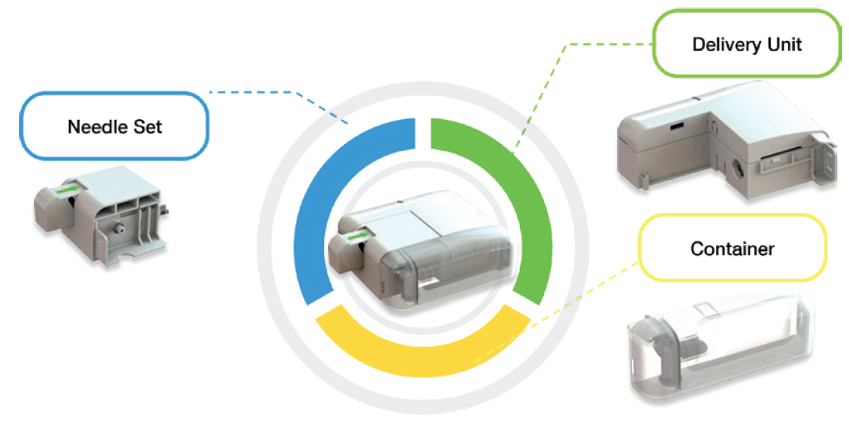
CCBIO’s Felice Dose on-body wearable device
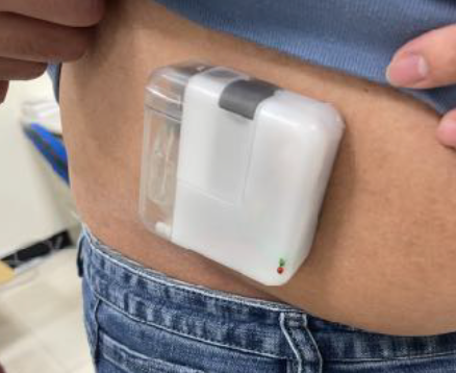
CCBIO’s Felice Dose on-body wearable device prototype worn on a person
Fabio Chianelli, Chief Executive Officer of PharmaTher, commented: “We are excited to work with CCBIO, a leader in wearable delivery injectors, to develop and commercialize a proprietary wearable ketamine delivery device that aims to change the way ketamine is given to potentially reduce the treatment burden of patients and healthcare providers. In addition, the current, evolving, and future therapeutic use of ketamine is dependent on the control of ketamine dosing to deliver pharmacokinetic profiles specific to each indication. Therefore, our goal is to become a leader in specialty ketamine solutions, which include injectable, intravenous, microneedle patch and now a ketamine wearable device that each serve a unique solution to enable tailored pharmacokinetic profiles for various mental health, neurological, and pain disorders.”
Edgar Yeh, Vice General Manager of CCBIO commented: “We are thankful to have the opportunity to work with PharmaTher to provide a patient-centric ketamine on-body injector device for patients. CCBIO’s in-house design, V/V, RA, and patenting capabilities enable us to produce competitive high-end medical devices for multiple applications. We are eager to partner with PharmaTher to generate and market a convenient ketamine wearable device that supports the needs of patients and caregivers using ketamine therapies.”
CCBIO’s Felice Dose is a delivery motor system-based on-body subcutaneous injector device that can deliver various drug volumes, concentrations and viscosities over variable- and long-duration injections. Felice Dose can deliver a stable dose of drug per unit time under discontinuous pressure, reducing the swelling underneath the skin during injection and thus decreasing patient pain. Discontinuous pressure for a high-dose container is important. Otherwise, if a drug delivery system continually places a positive linear compression pressure on the container, pressure will accumulate and increase patient pain during the injection process. In addition, the delivery motor system can adapt to the primary drug container, providing a very stable displacement unit for the drug, and the duration for filling and delivery can be programmed to suit the drug formulation. The Felice Dose user interface includes options for WiFi, near-field communication and Bluetooth connectivity, an LED display, fully-customizable programmability, a robust delivery motor system and a lithium battery. The device’s smart program functionality can help patients take control of their treatment, make their daily lives easier and more comfortable, and reduce the need for hospital visits and the involvement of healthcare providers.
Under the agreement, PharmaTher will cooperate with CCBIO to develop PharmaTher’s wearable ketamine delivery device. CCBIO will be responsible for the development and clinical manufacturing activities and support PharmaTher’s regulatory submissions.
About CC Biotechnology Corporation
Founded in 2012, CCBIO is a Taiwan-based medical device designer and manufacturer of wearable, pen and auto injectors and follows ISO13485, FDA21 CFR820 and GMP requirements.
About PharmaTher Holdings Ltd.
PharmaTher Holdings Ltd. (OTCQB: PHRRF) (CSE: PHRM) is focused on the development and commercialization of specialty ketamine pharmaceuticals for mental health, neurological, and pain disorders. Learn more at
PharmaTher.com.